Wouldn't compare 747 metal pairing to the VX. 747 has a much more proactive maintenance and no exposure to salt.
I also wouldn't rely on coating as the clamping force of very rough surfaces will likely penetrate any coating.
Ultimately, you're trying to design a problem away that doesn't exist. The corrosion resistance of the spacers is not the issue (and only exist to create a barrier between the subframe and chassis), they are only replaced as they are a cheap item to replace and often deform as the aluminium chassis builds up aluminium oxide at the chassis-spacer interface. Just spec a like-for-like part
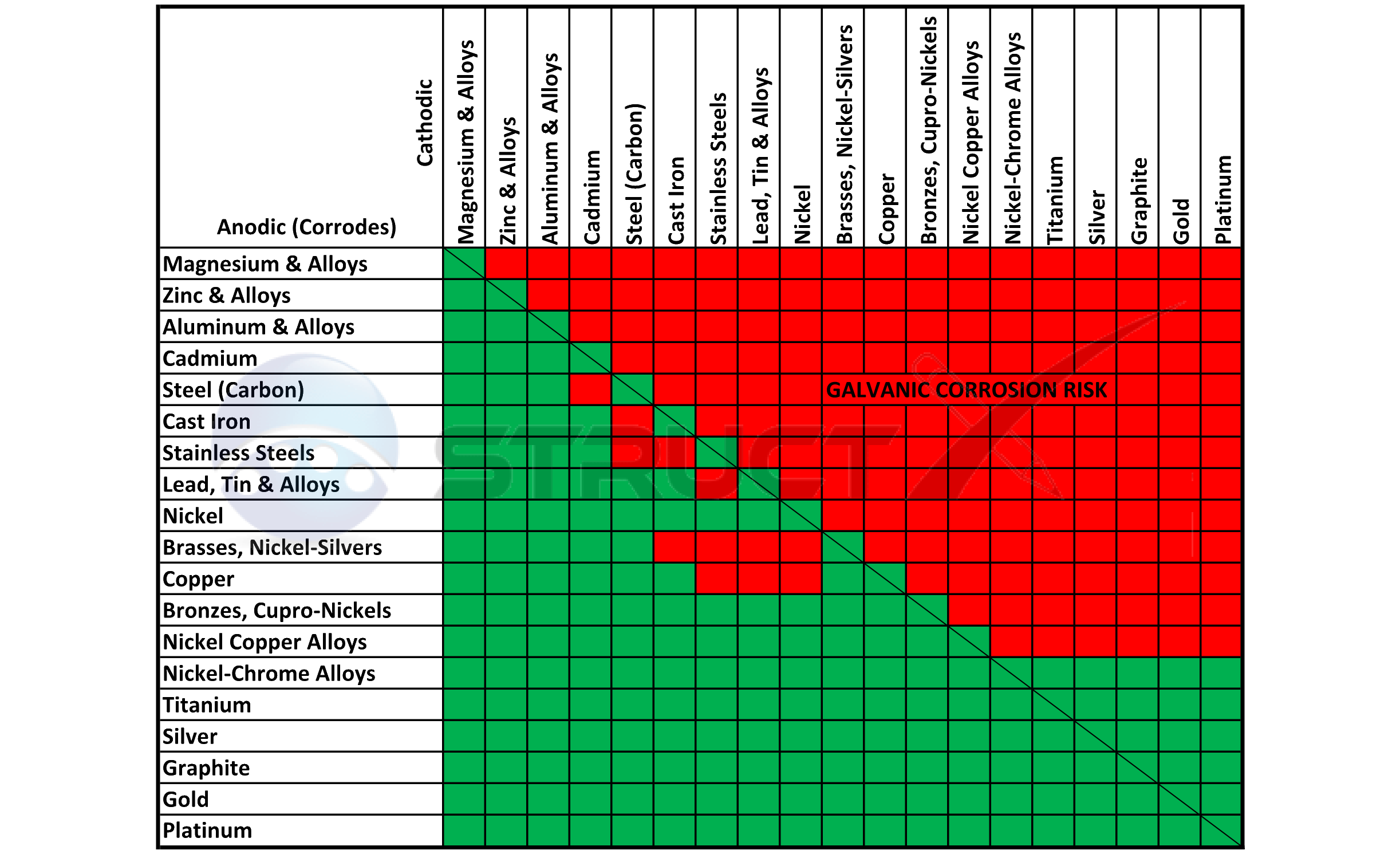
True though the principal is the same, to get as many of the components as close to each other as possible in the Galvanic table either by base material or plating though both is a superior solution. If you are going to have to plate the steel option anyway then it is no different to plate a stainless version.
400 series active stainless is almost identical to steel with the advantage of not decaying itself.
Always worth looking at improving the OE if possible, most OE material decisions were heavily based on cost.
If the material cost is going to be £60p for steel and £1.20 for 400 SS then it would be a no brainer as in the total production costs this would be negligable.
Might be worth a little research on the latest compounds as there is probably something more suited with superior capabilities available today.
Some of the latest off shore use compound technology lasts for years.
If you can design the problem out altogether why wouldn't you try?
Anyone know why the plate is bent?
The principle isn't the same. Stainless steel is significantly more active than steel when coupled to aluminium. If the coating is damaged (visual inspection of the plates will confirm it will be penetrated) then steel has a much weaker galvanic couple than stainless steel with aluminium.
Suspect the plate is bent to aid seating before mounting the subframe.